Corrective and Preventive Action (CAPA) emerges as a vital component for the pharmaceutical industry’s approach to quality management, directly impacting compliance with the International Conference on Harmonisation (ICH) Q10 Pharmaceutical Quality System (PQS).
The ICH Q10 framework is designed to enhance product quality, consistency, and regulatory adherence across the global pharmaceutical industry. CAPA plays a fundamental role in achieving these objectives by systematically identifying, addressing, and preventing quality issues. This article explores the significance of CAPA in achieving ICH Q10 compliance, providing industry professionals with insights into optimizing their CAPA processes for enhanced regulatory compliance and product quality.
Understanding ICH Q10 and Its Quality Objectives
ICH Q10 is an internationally harmonized guideline aimed at establishing a comprehensive pharmaceutical quality system as a model for ensuring uniform product quality throughout the life cycle. It complements existing Good Manufacturing Practice (GMP) requirements and aligns with other regulatory expectations, such as those from the U.S. FDA and European Medicines Agency (EMA).
As part of the tripartite guideline, alongside ICH Q8 and ICH Q9, ICH Q10 contributes to a comprehensive framework for pharmaceutical product development and manufacturing. ICH Q10 applies to systems that support the production of drug substances and drug products, including biotechnology and biological products, across their entire lifecycle. These systems oversee critical processes such as formulation development, drug manufacturing, documentation retention, quality control, change management, and CAPA systems.
Now, the key objectives of ICH Q10 include:
⇒ Achieving Product Realization – Ensuring consistent quality in drug development and manufacturing.
⇒ Maintaining a State of Control – Implementing robust monitoring and control mechanisms to detect and address quality risks.
⇒ Facilitating Continuous Improvement – Encouraging pharmaceutical companies to enhance their processes for better efficiency and regulatory compliance.
To achieve these objectives, ICH Q10 emphasizes four main enablers: process performance monitoring, change management, knowledge management, and CAPA. Among these, CAPA stands out as a proactive and reactive approach to quality management, ensuring ongoing compliance and product integrity.
Delving into the Components of ICH Q10 Pharmaceutical Quality System Model
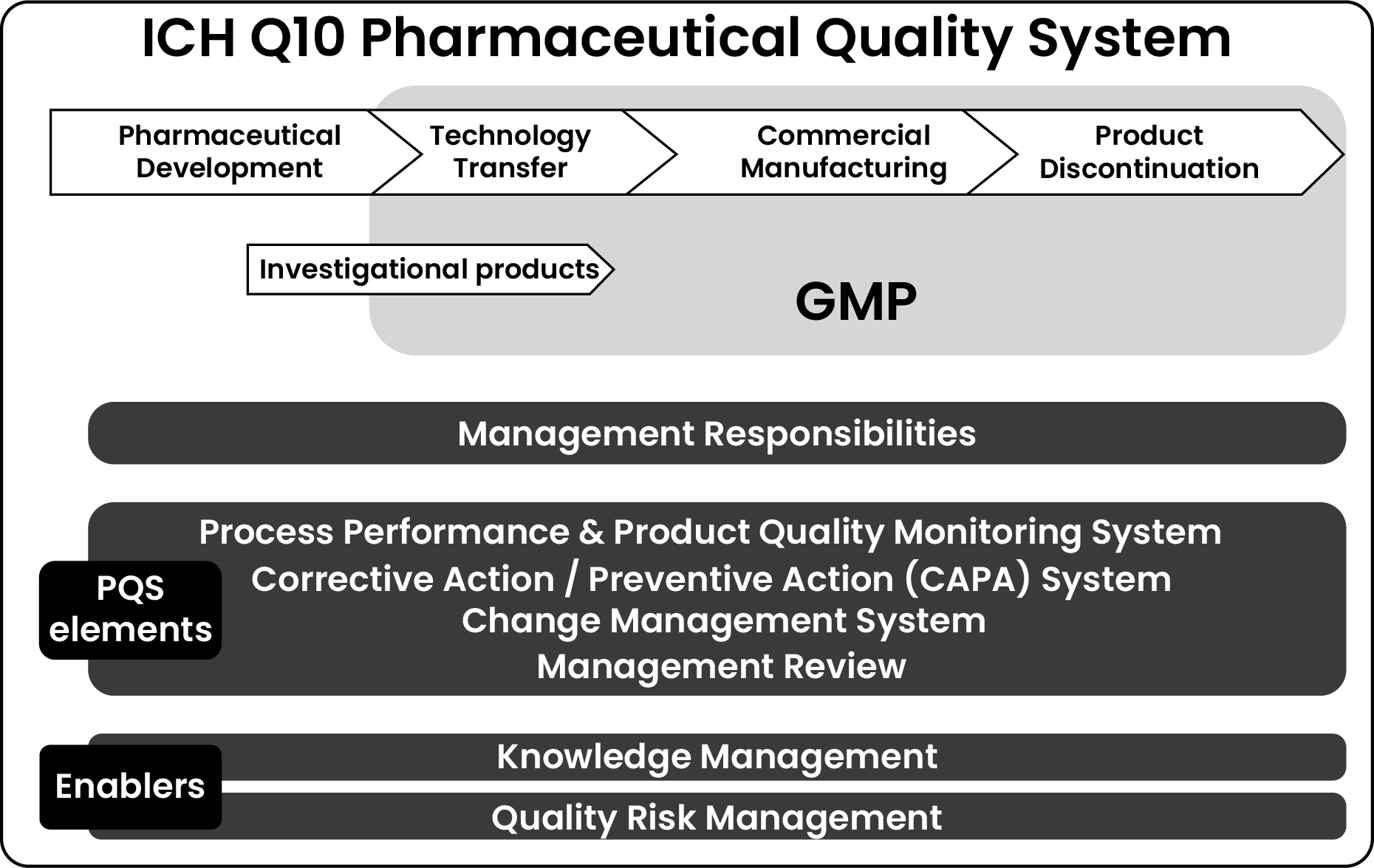
4 Key Stages of ICH Q10 PQS
ICH Q10 is designed to be established across the following four key stages:
a) Pharmaceutical Development
This phase involves designing the product and defining a robust manufacturing process to meet regulatory requirements, ensure drug efficacy, and address patient needs.
- Develop drug substances and formulations, including container-closure systems.
- Plan and execute investigational product manufacturing for clinical trials.
- Establish a scalable and efficient manufacturing process.
- Develop analytical methods to assess product quality and performance.
b) Technology Transfer
The goal of this phase is to transfer product and process knowledge between R&D and commercial manufacturing.
- Transition products from development to production while maintaining consistency.
- Transfer manufacturing processes between different sites or facilities.
- Ensure process validation, establish a control strategy, and prepare for continuous improvement.
c) Commercial Manufacturing
This phase focuses on ensuring consistent production, maintaining quality, and enabling continuous improvement.
- Manage raw material acquisition, equipment, facilities, and utilities.
- Conduct production, packaging, labeling, and quality assurance (QA) activities.
- Implement process monitoring systems to maintain a state of control.
- Perform regular quality reviews and corrective/preventive actions (CAPA) to address deviations.
d) Product Discontinuation
A structured approach is required to phase out a product while ensuring compliance and risk mitigation.
- Ensure document and sample retention for future reference.
- Conduct ongoing product assessments and report to regulatory agencies.
- Manage product withdrawal in a controlled manner to prevent unintended risks.
4 Essential Elements of ICH Q10 PQS
ICH Q10 defines the following elements that contribute to an effective PQS:
a) Process Performance & Product Quality Monitoring System
A risk-based approach is applied to monitor critical parameters that affect product quality.
- Ensures consistency in manufacturing performance.
- Uses data analytics to detect trends and prevent failures.
b) Corrective Action/Preventive Action (CAPA) System
A structured system to identify, investigate, and correct deviations.
- Helps mitigate potential risks before they affect product quality.
- Ensures timely resolution of non-conformities and complaints.
c) Change Management System
Manages modifications to processes, equipment, and documentation.
- Ensures controlled implementation of changes.
- Prevents disruptions to product quality and supply chain.
d) Management Review
Regular evaluations by senior leadership to assess the effectiveness of the PQS.
- Identifies areas for improvement and regulatory updates.
- Aligns business goals with quality objectives.
2 Enablers for an Effective PQS
ICH Q10 emphasizes two enablers to support a robust quality system:
a) Knowledge Management
A structured approach to capturing, sharing, and utilizing information across the product lifecycle.
- Enhances decision-making with a scientific, data-driven approach.
- Facilitates technology transfer between R&D and manufacturing teams.
b) Quality Risk Management (QRM)
A systematic process to assess, control, communicate, and review risks.
- Ensures risk-based decision-making across development, manufacturing, and distribution.
- Helps maintain regulatory compliance while optimizing efficiency.
Role of a Systematic CAPA Approach in ICH Q10 Compliance
CAPA is a structured methodology used to investigate, correct, and prevent quality issues within the pharmaceutical industry. It encompasses two core elements:
A. Corrective Actions – Addressing non-conformities and deviations by identifying the root cause and implementing necessary fixes.
B. Preventive Actions – Taking proactive measures to eliminate potential risks before they manifest into quality issues.
⇒ How a Strong CAPA Process Facilitates ICH Q10 Compliance
The effectiveness of an adequate CAPA process in achieving ICH Q10 compliance lies in its ability to:
- Detect and mitigate risks to ensure product safety and efficacy.
- Facilitate a culture of continuous improvement within the organization.
- Strengthen regulatory compliance by demonstrating a robust quality management system.
⇒ How a CAPA System Assists in Continuous Improvement & Knowledge Management
ICH Q10 promotes continuous improvement as a fundamental principle of pharmaceutical quality management. CAPA fosters this principle by enabling organizations to:
- Collect and analyze historical quality data to identify improvement opportunities.
- Leverage insights from past deviations to enhance process control and reliability.
- Encourage cross-functional collaboration between manufacturing, quality assurance, and regulatory teams.
Knowledge management—another key enabler of ICH Q10—benefits significantly from CAPA. Lessons learned from CAPA investigations help build an organizational knowledge base that informs future quality decisions and risk mitigation strategies.
⇒ How CAPA Management Help Meet Regulatory Expectations
Regulatory agencies emphasize the importance of CAPA in pharmaceutical quality systems. Key regulatory expectations include:
Thorough Documentation – All CAPA investigations and actions must be well-documented and accessible for audits.
Data-Driven Decision Making – CAPA effectiveness should be evaluated using objective quality metrics.
Timely Resolution – Delays in CAPA implementation can result in compliance violations and regulatory scrutiny.
CAPA is a core mechanism in achieving ICH Q10 compliance, ensuring pharmaceutical products meet the highest quality standards. By prioritizing CAPA as a cornerstone of quality management, organizations can successfully navigate the complexities of ICH Q10 and strengthen their commitment to pharmaceutical excellence.
Drive ICH Q10 Compliance With Intelligent CAPA Solutions: What Sets Smart CAPA Apart
Keeping up with ICH Q10 compliance can be a challenge for pharmaceutical manufacturers. Managing deviations, investigating root causes, implementing corrective actions, and ensuring regulatory documentation—all while maintaining operational efficiency—can quickly become overwhelming.
What if you could transform CAPA management into a proactive and self-run process that keeps compliance on track without the hassle?
Here’s a quick list of software that helps you do just that and achieve ICH Q10 Compliance:
Now, how Smart CAPA stands out you wonder?
While the above platforms offer excellent capabilities, Smart CAPA stands out with a focused, compliance-driven framework that makes ICH Q10 adherence intuitive and smarter for pharmaceutical companies.
Smart CAPA is designed in a way that enables pharmaceutical companies to identify, track, and resolve quality issues faster while contributing to ensuring effective compliance with specific ICH Q10 requirements.
Here’s how Smart CAPA directly contributes to each pillar of ICH Q10:
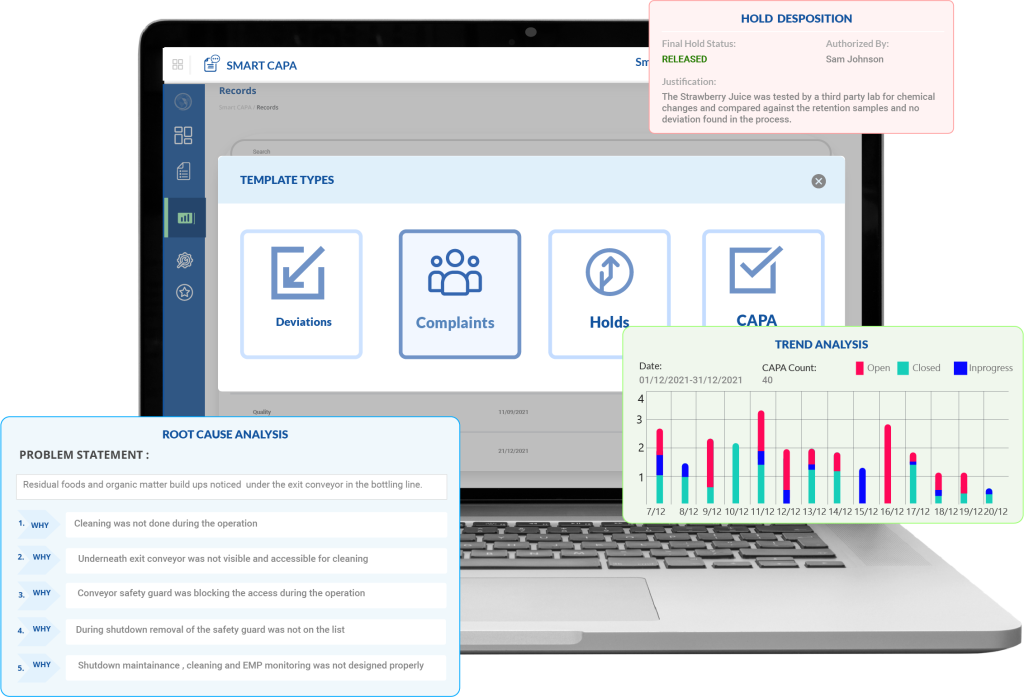
Automated CAPA Management to Ensure Immediate Action and Structured Follow-Up
Smart CAPA eliminates delays in identifying and responding to quality issues by:
- Automatically capturing quality events from integrated modules (like deviation, complaint, or audit findings) and initiating a CAPA without manual intervention.
- Triggering predefined workflows for investigation, task assignment, approvals, and escalations—ensuring that each step follows a regulatory-compliant path.
- Assigning responsibilities with due dates and reminders, making it impossible for CAPA steps to be missed or delayed—especially crucial during regulatory inspections.
Example: When a deviation is logged during batch release, Smart CAPA auto-initiates a CAPA and assigns investigation tasks to the QA lead, all while tracking every action with timestamps.
Structured Root Cause Analysis Tools to Prevent Repeat Issues
ICH Q10 expects true root cause identification—not surface-level fixes. Smart CAPA facilitates this by:
- Embedding interactive RCA tools like the 5 Whys, Fishbone Diagram, and FMEA directly into the CAPA form—no switching between systems or attaching offline documents.
- Allowing teams to collaborate in real time within these tools and record multiple potential causes before narrowing down the validated root.
- Capturing every step of the RCA with user stamps, creating a traceable reasoning path for auditors and internal reviews.
Example: A recurring contamination issue is analyzed with Fishbone and validated via FMEA. The high-priority cause (poor cleaning SOP adherence) is logged with risk scoring and forms the basis for targeted corrective actions.
End-to-End Traceability and Documentation Aligned with Regulatory Expectations
Documentation gaps are a common cause of FDA 483s and warning letters. Smart CAPA helps avoid this with:
- A centralized CAPA repository where each record is version-controlled, searchable, and linked to related quality events and actions.
- Full traceability across the CAPA lifecycle—from issue identification to action verification and closure—with electronic signatures and time-stamped entries compliant with FDA 21 CFR Part 11.
- Built-in document linking to associate SOPs, batch records, and training logs with each CAPA, proving implementation and effectiveness during audits.
Example: During an audit, QA pulls up a CAPA related to a failed stability test—showing the linked investigation report, root cause analysis, revised SOP, and post-training verification in under 2 minutes.
Real-Time Insights to Drive Ongoing Quality System Improvements
ICH Q10 is not just about reacting to problems—it’s about building a system that evolves. Smart CAPA supports this vision by:
- Providing dashboards that track open vs. closed CAPAs, overdue actions, recurring root causes, and department-wise performance.
- Offering customizable reports to monitor CAPA effectiveness and identify systemic issues—helping QA and compliance teams proactively address trends before they escalate.
- Generating automated compliance reports for management reviews, so leadership has visibility into how the QMS is performing across functions.
Example: CAPA trend analysis reveals that most deviations are arising from one production line. Management uses this data to schedule a targeted audit and retraining, preventing future non-conformances.
While other solutions provide CAPA functionality, Smart CAPA can be carefully catered to serve the very spirit of ICH Q10. From initiation of root cause analysis to traceable action verification and continuous monitoring—it delivers a complete, tech-enabled, and audit-ready CAPA system for your industry.
If your goal is to simplify compliance while strengthening your pharmaceutical quality system, Smart CAPA gives you the structure, clarity, and speed to make it happen.